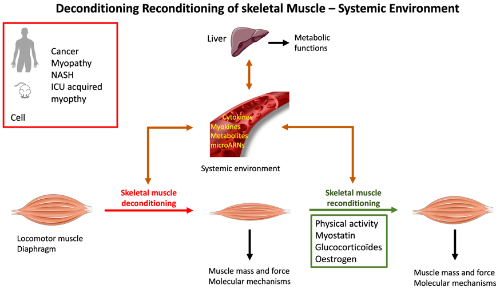
DeReM-SE project
Conceptual framework of the scientific project of the DeReM-SE team
Cancer
Cachexia is an extremely rapid and severe form of skeletal muscle deconditioning with particularly high prevalence in pulmonary, pancreatic and digestive cancers (Teunissen et al. 2007). Furthermore, therapeutic interventions (excision, chemotherapy, radiotherapy) can exacerbate cachexia. The molecular mechanisms involved in cancer cachexia are now relatively well-understood (Argiles et al. 2014). Previous work by our team has notably highlighted the existence of an imbalance between the synthesis and degradation of skeletal muscle proteins (Gallot et al. 2014). However, the systemic regulators triggering the catabolic program skeletal muscle are poorly understood. The role of glucocorticoids and the hypothalamic-pituitary-adrenal axis will be studied in ApcMin/+ mice, a mouse model of the intestine and colon cancer (collaboration with Team ATPA). As the skeletal muscle tissue is a privileged metabolic partner of the liver (Argiles et al. 2018), the systemic effects of skeletal muscle deconditioning on hepatic metabolism will be also studied. We have shown that myostatin gene invalidation of the gene for, a negative regulator of muscle mass (Durieux et al. 2007), is an effective strategy to prevent cachexia in ApcMin/+ mice (Gallot et al. 2014). The effects of preventing muscle cachexia by myostatin gene invalidation will also be determined in relation to the regulation of hepatic metabolism. The biological function of glucocorticoids in this context will also be explored.
The effects of cancer on skeletal muscle recovery following surgery will also be explored in a population of patients with cancer of the stomach, intestine, appendix, colon, and rectum (MYOCAC ClinicalTrial Identifier NCT03172403). The biological analysis of the muscle biopsy obtained during surgical resection will be coupled with an analysis of skeletal muscle mass, muscle function and blood samples up to 6 months after the surgical intervention (collaborations ATPA & PAF teams).
Intensive care unit acquired muscle weakness
Skeletal muscle deconditioning acquired in intensive care unit (ICU), also ICU-acquired muscle weakness, is a frequent complication in ICU associated with high mortality and morbidity, sepsis being an aggravating condition this syndrome (Griffiths and Hall 2010). Previous work by our team has well described the time course of the mechanisms involved in skeletal muscle deconditioning in a murine model of sepsis (Morel et al. 2017). However, the systemic factors upstream of the activation of this catabolic program are still poorly understood. The role of glucocorticoids in skeletal muscle deconditioning in sepsis will be determined. The effects of myostatin gene invalidation will also be studied.
The early diagnosis of skeletal muscle deconditioning in ICU is an important factor for clinicians since it will allow orienting and adapting the therapeutic practice. The analysis of skeletal muscle deconditioning at the patient's bed (muscle morphometry and strength, systemic environment) will allow the development of innovative and adapted rehabilitation techniques (muscle electrostimulation, cycloergometer) to improve the quality of care, the prognosis and the post-resuscitation patients’ quality of life (collaboration PAF Team).
The muscular deconditioning acquired in ICU also affects the respiratory muscles. While our work in animals has demonstrated a catabolic resistance to sepsis for the respiratory muscles when compared to locomotor muscles (Morel et al. 2017), the situation is very different in human patients. The mechanical ventilation, which can supplement ventilatory failure (which sometimes lasts several weeks) in septic patients, can also become a major cause of respiratory muscles deconditioning (Levine et al. 2008). The purpose of the SEPTIC study (ClinicalTrials Identifier NCT03371602) is to determine the combined effects of mechanical ventilation and sepsis on the human diaphragm. The molecular mechanisms of muscle deconditioning will be explored in the diaphragm muscle of control patients (non-septic programmed gastrectomy, esophagectomy, pancreatectomy, hepatectomy), septic patients (abdominal or thoracic surgery in a septic context: peritonitis, mediastinitis, pleural abscess), ventilated brain-dead patients, and ventilated patients under controlled mechanical ventilation after an abdominal or thoracic surgery in a septic context.
Fatty liver diseases. Liver and skeletal muscle tissues are privileged metabolic partners through the regulation of glucose, lipid or amino acid, metabolism, but also via cytokinic interactions. Here, the effects of a metabolic liver pathology on skeletal muscle will be considered. Nonalcoholic steatohepatitis (NASH) is characterized by an excessive accumulation of triglycerides in the liver and is associated with a marked inflammatory state (De Bandt 2018). While patients with NASH experience skeletal muscle deconditioning (De Bandt 2018), the effects of this inflammatory condition on skeletal muscle tissue and the liver-skeletal muscle organ cross-talk are not completely understood. The molecular mechanisms of skeletal muscle deconditioning will be explored in the quadriceps muscle of NASH patients (project in preparation). Primary hepatocyte-myotube co-culture models will be used to determine the effects of the hepatocyte-modulated environment on skeletal muscle tissue.
Congenital myopathy
Unlike previous pathologies for which skeletal muscle deconditioning occurred in response to the evolution of a non-muscular pathology, congenital myopathies have a genetic origin with a strongly marked phenotypic manifestation in skeletal muscle tissue. Centronuclear myopathy linked to the mutation of the dynamin 2 gene (dnm2) is a pathology characterized by a loss of skeletal muscle mass and force. The R465W mutation in KI dnm2 mouse is the mouse model of this pathology (Durieux et al. 2012). The effects of myostatin gene invalidation on skeletal muscle of KI dnm2 mouse will be determined. In addition, preliminary data from our team highlighted the existence of mitochondrial structural anomalies, that may reflect an alteration in mitochondrial homeostasis in the muscle fibers of KI dnm2 mice. This work will be continued by analyzing mitochondrial metabolism and the mechanisms of mitochondrial fusion and fission. The effects of endurance training on mitochondrial homeostasis and improvement of the muscle phenotype of KI dnm2 mice will be determined. The therapeutic impact of an oestrogen receptor-alpha targeting strategy will be also evaluated.