VBRBC project
1. Hemoglobinopathies and other RBC disorders
1.1. Blood rheology, genetic factors and microcirculation
Similar to the preceding laboratory accreditation (Connes et al, 2016 ; Renoux et al, 2016 ; 2017 ; 2019 ; Ballas et al, 2018), we will continue working on the involvement of blood rheological alterations and genetic factors in the pathophysiology of SCD. We plan to use the oxygenscan technique (ektacytometry with oxygen gradient; Rab et al, 2018; 2019) to screen for the ability of SCD RBC to sickle under deoxygenation in the Lyon cohort, as well as in two other cohorts (Utrecht, The Netherland & Houston, USA) to test the usefulness of the point-of-sickling (POS) criteria to predict SCD severity and the occurrence of acute and chronic complications (hemolytic and vaso-occlusive like complications; Eurostars-H2020-SCOOP project). We will test the influence of classical genetic modulators of SCD severity (alpha-thalassemia, G6PD deficiency, HbF-QTL), as well as new potential genetic modulators (NOS1 and 2, APOL1, CD36, genes involved in oxidative stress….) on RBC rheology. We will also test the effects of various classical therapies (hydroxyurea, transfusion) and new molecules (collaboration with Hartis Pharma company) on RBC rheology, notably the POS parameter, to improve management of SCD patients and identify potential new therapies. The use of microfluidic approaches will also help defining different RBC flowing signatures in SCD, that could help predicting the clinical severity of the patients and improve clinical management.
1.2. Eryptosis, microparticles, vascular function and physical activity in SCD and hereditary spherocytosis
SCD is characterized by RBC abnormalities. Studies performed in the last two decades also demonstrated severe alterations in the vascular function, which participates in the occurrence of several acute and chronic complications. Enhanced oxidative stress and inflammation, decreased nitric oxide (NO) bioavailability and accumulation of high concentration of microparticles have been proposed to contribute to the development of chronic vasculopathy in SCD (Charlot et al, 2016 ; Moeckesch et al, 2017; Nader et al, 2020). We will test the association between these factors and the micro- and macro-circulatory function in adults SCD (VASCU-DREPA project in Guadeloupe). In addition, we will address the same question in another RBC disease, called hereditary spherocytosis (Vascu-Sphero, Lyon). Moreover, because chronic physical activity may modulate vascular function in various chronic diseases, we will test 1) the associations between the level of physical activity and vascular function in SCD (VASCU-DREPA project) and 2) the effects of regular physical activity (training programs and promotion of physical activity) on the vascular function and several biomarkers in SCD (DREPA-SPORT project in Lyon, DREPA-AP project in Senegal). Moreover, recent studies from our lab also demonstrated profound microcirculatory remodeling of the skeletal muscles as well as muscle structural and energetics abnormalities in SCD (Ravelojaona et al, 2015). Impaired muscle functioning could participate in exercise intolerance of SCD patients. For these reasons, the impact of SCD on muscle function during localized endurance will be tested (DREPA-MUSCLE project, Lyon, collaboration with SPIP team of the LIBM). Among the other candidates that could modulate vascular function, obstructive sleep apnea (OSA) has been demonstrated to cause vascular dysfunction in the general population and metabolic disease. The prevalence of OSA is not well described in SCD but few studies have suggested that it could reach more than 40% in adults SCD. The DREPA-APNEE project (Lyon) will investigate the role of OSA in the modulation of the vascular function, clinical severity and several biomarkers known to play a role in the pathophysiology of SCD (blood rheology, inflammation).
In the recent years, we started focusing on eryptosis and RBC microparticles genesis which are both increased in SCD compared to healthy individuals. Our first results demonstrated that both oxidative stress and NO were able to modulate eryptosis (RBC phosphatidylserine exposure, accumulation of Ca2+ and reactive oxygen species) but only oxidative stress impacted the release of microparticles by sickle RBCs. We now plan to test the effects of RBC-derived microparticles isolated from SCD patients on endothelial cells phenotypes and activation. We will explore the TRL-4 and the phosphatidylserine pathways to better understand the cascade of events that could link RBC microparticles and endothelial/vascular dysfunction in SCD. Moreover, a growing interest has been recently devoted to the study of Piezo1, a mechanosensitive protein, in the context of hereditary xerocytosis. When stimulated, this protein is able to make calcium entering in the RBCs. Indeed, we suspect that Piezo1 could be a strong modulator of eryptosis, RBC dehydration (through Gardos channel activation) and MP-genesis and future works of our team will test these hypotheses (collaboration Lyon-Roscoff-Guadeloupe).
1.3. Sickle cell trait and type 2 diabetes
We previously demonstrated that the combination of sickle cell trait (SCT) with type 2 diabetes resulted in a severe vascular dysfunction and increased the risks for vascular complications (retinopathy, arterial hypertension, glomerulopathy) compared to individuals with diabetes (Diaw et al, 2015 ; Skinner et al, 2018). We have found that AGEs could be involved in the vascular dysfunction, and in-vitro experiments incubating endothelial cells of the microcirculation with plasma of the patients support a key role of oxidative stress. We now plan to use metabolic approach to further investigate the pathways that could be involved in this higher vascular dysfunction described in combined SCT-diabetes individuals. Moreover, we recently demonstrated greater vascular dysfunction in Townes SCT mice with diabetes compared to healthy Townes mice, Townes SCT mice and Townes diabetic mice (Skinner et al, 2019). In collaboration with the UMR 5305 CNRS Lyon (Drs B Fromy and D Sigaudo-Roussel). We now plan to test the involvement of COX-2 and inflammation.
2. Exercise/Hypoxia
In the previous years, we conducted several studies to test RBC and vascular adaptations in the context of exercise or altitude exposure. From a physiological point of view, the hematological changes induced by altitude exposure, and to a lesser extent by acute exercise, are at the opposite of those observed in SCD or spherocytosis (polycythemia versus anemia). However, these extreme situations share common pictures: a) hyperleucocytosis during acute intense exercise and in SCD, b) blood hyperviscosity during exercise, in polycythemic individuals and in some SCD patients (notably in SCD patients with SC disease), c) highly vasodilated arteries in high altitude residents and SCD patients, d) the loss of vascular reserve in high altitude residents and SCD patients, e) the presence of chronic inflammation in high altitude residents with chronic mountain sickness and SCD patients. Moreover, some healthy endurance athletes develop exercise-induced hypoxemia while high altitude residents are exposed to chronic hypoxemia and some SCD patients may be hypoxemic. Hypoxia (acute and chronic) seems to be a common denominator in all these situations but the consequences are extremely different. Understanding the relationships between the hematological and blood rheological changes, and the vascular responses in each of these situations will allow us to better understand the pathophysiological mechanisms at the origin of some acute and chronic clinical complications observed in these different populations.
In the past, we have extensively described the hematological and blood rheological changes observed in response to a short acute exercise (Connes et al, 2013 ; Simmonds et al, 2013). However, the RBC responses to extreme endurance exercises are unknown and part of the future studies will address this point. Moreover, some endurance athletes use hypoxic stimulus to stimulate erythropoietic response and increase hematocrit and hemoglobin mass, in order to improve their performance when they return to sea level. However, about one-half of endurance athletes may develop acute hypoxemia and adaptative responses to hypoxic exposure are unknown. We will test the impact of several days at moderate altitude on hematological, vascular and exercise parameters in endurance athletes with and without exercise-induced hypoxemia. Finally, we recently participated to the EXPEDITION 5300 project http://expedition5300.com This project was lead by Dr S Verges (UMR Inserm HP2, Grenoble) and was performed in the highest city of the world (La Rinconada, Peru, 5100-5300 m above sea level). We explored the vascular function, hematological, blood rheological and biological parameters in high altitude residents to better understand the mechanisms that could explain why about 25% of the populations leave with a chronic mountain sickness (CMS). We demonstrated a key role of blood viscosity in the occurrence of CMS. The first results also shown that vascular reserve could be limited in individuals with CMS because of the presence of highly dilated arteries. We now plan to study: 1) the mechanisms at the origin of this particular vascular response and 2) the effects of two therapies (acetazolamide and statin) on CMS and vascular/biological responses.
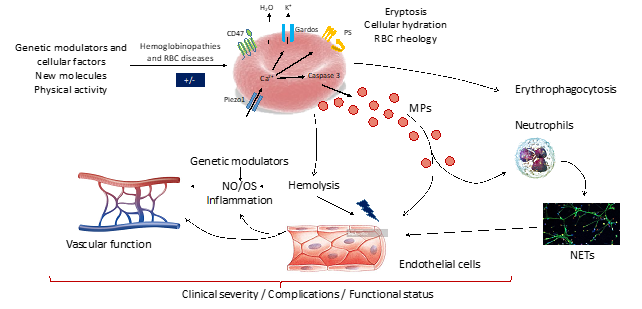
Figure 4. Schematic representation of the “Hemoglobinopathies and RBC disorders” topic